The word "nylon" for a person who is not familiar with the science behind the plastics instantly reminds: socks, velcro, reeds for guitar, fishing line and different types of clothing. But the application of polyamides is not limited to simple parts and textile yarn, far from it, polyamides are the main engineering plastics in the market. To give you an idea, in 2002 (sorry for delayed data) the total world consumption of polyamide amounted to 464,000 tonnes!
Polyamides are not polymerized from the same substances, but they all have the amide functional group (CONH). Some of these polymers are obtained from two basic materials, each with a number of carbon atoms corresponding to the carbon number of the polyamide. PA 6.6 for example, is so called because of hexamethylenediamine and adipic acid, its raw materials, having 6 carbon atoms each. Since the PA 6 is polymerized from caprolactam has 6 carbon atoms.
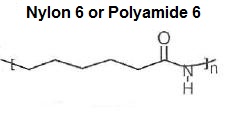 Chemical structure of Polyamide 6
Chemical structure of Polyamide 6
The polar amide groups (CONH) directly influence the properties of polyamides, as much shorter the distance between these groups as better its mechanical and thermal properties. However, resistance to water absorption decreases due to increased hydrogen bonding. The best examples are the PA 6 and PA 6.6 which are most used in the injection molding of engineering parts and have high values of water absorption when compared to PA 6.10, 11 and 12.
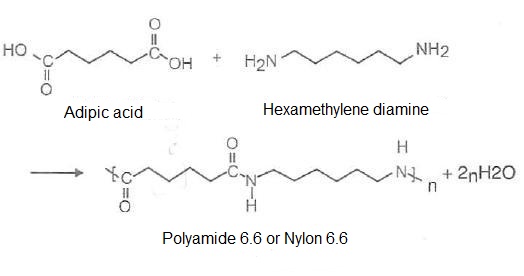 Chemical structure of Polyamide 6.6
Chemical structure of Polyamide 6.6
In some cases, drying of the PA is required prior to injection molding to prevent the appearance of stains on parts, differences in polymer melt viscosity and other typical problems presented by wet plastics, particularly in cases of recycled materials. Drying may be performed through tray dryers, circulating air dryers or dehumidifiers hoppers over a period of 4 hours at 176°F (80°C) (PA 6 and 6.6). Leave this much beyond the recommended time can cause yellowing of polymer of natural color. Preheating also facilitates the processing, making part of the cylinder work, preventing the occurrence of burn points. Drying can be eliminated from the process depending on factors such as geometry and application of part; type and capacity of the machine; cost due to the electric power expense, time and drying capacity. Injection molding tests and costing may be necessary to verify whether the removal of this step of the process really pays off.
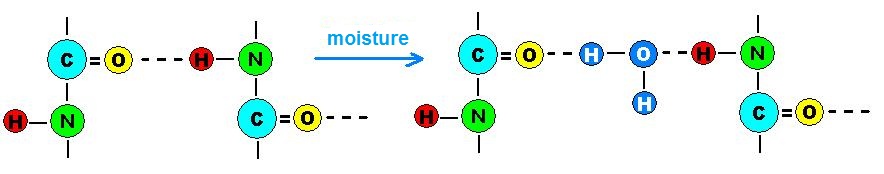 Formation of hydrogen bonds in polyamides
Formation of hydrogen bonds in polyamides
In most cases, after the molding injection is done the opposite way: the immersion of parts in a container with water to make this acts as a plasticizer in the polymer, separating molecular chains and decreasing crystallinity and glass transition temperature (Tg) of a value of about 122°F (50°C) to 32°F (0°C), i.e., the part that might need to overcome 122°F not to stand so hard and brittle as glass will need only 32°F. This makes a rigid and fragile piece becomes tough and resistant to impact after hydration. The time required for this process step, or its elimination, varies with the following factors: mechanical properties required by the application part, the presence of metal inserts that can become loose after hydration and available time between the production of the part and its packaging. It is recommended that the moisture content in parts made from polyamide is between 1 and 2%.
Polyamides have a very good acceptance of the incorporation of glass fiber, mineral fillers, pigments, stabilizers, lubricants and impact modifiers.
Features:
- High resistance to fatigue
- Good impact resistance
- High melting temperature
- Low coefficient of friction
- Resistance to weathering
- Great mechanical properties
- High flow
- Impervious to gases
- Low resistance to inorganic acids (nitric, hydrochloric, sulfuric etc.)
- Low resistance to aromatic alcohols (benzyl alcohol, phenols, cresols etc.)
- Hygroscopic
Applications:
Gears, automotive parts, bushings, seals and clothes.
Polyamide 6
Melting point: 428°F (220°C)
Glass transition: 113°F (45°C)
Poliamida 6.6
Melting point: 482°F (250°C)
Glass transition: 122°F (50°C)
Bibliography:
HARPER, Charles A.; PETRIE, Edward M. Plastics Materials and Process: A Concise Encyclopedia. Hoboken: John Wiley & Sons, Inc., 2003.
CANEVAROLO JR., Sebastião V. Ciência dos Polímeros: Um texto básico para tecnólogos e engenheiros. 2.ed. São Paulo: Artliber Editora, 2002.
WIEBECK, Hélio; HARADA, Júlio. Plásticos de Engenharia: Tecnologia e Aplicações. São Paulo: Artliber Editora, 2005.
© 2010-2025 - Tudo sobre Plásticos.
All rights reserved.
Home
-
Privacy policy
-
Contact